The Phage is a Lonely Hunter
New research reveals that bacteriophages use slow, staccato movements to hunt bacteria on cell surfaces.
Sharks prowl the watery depths for their prey, lions stalk the tall-grass savannah, and bacteriophages have mucus. The thin layer of mucus that coats epithelial cells serves as the hunting ground for phages, viruses that kill and use bacteria to proliferate. Newly published research by scientists at San Diego State University finds that these phages use a novel hunting strategy known as “subdiffusive motion” to better their chances of finding bacteria within a mucosal surface.
The term “hunting” here is a bit of anthropomorphism. Viruses, including bacteriophages, aren’t alive in the strict biological sense. They have no means of independent locomotion. Instead, they float around in their environment and when they encounter a bacterium with the right kind of chemical receptors on its surface, they infect the host cell, hijacking its cellular replication machinery to produce more phages. It’s not purposeful hunting, but it’s effective.
These phages provide an unwitting secondary immune system to their mammalian hosts—which include humans—protecting them from harmful bacteria. Fostering helpful bacteriophages to counter bacteria forms the basis of an emerging type of medical treatment known as phage therapy. SDSU’s Viral Information Institute (VII) is a key player in this nascent field.
BAM kicks it up a notch
Previous research by SDSU scientists established the Bacteriophage Adherence to Mucus (BAM) model, which explored how phages attach to proteins in mucus, forming a symbiotic relationship between mammalian host and phage. Within the constantly secreting mucus layer, phages are free to drift about in search of bacteria.
Traditionally, phages were thought to move completely randomly through the mucus by normal diffusion, otherwise known as Brownian motion. But SDSU microbiologist Jeremy Barr, along with an interdisciplinary team of the university’s biologists, mathematicians, physicists and engineers, wondered if phages might have some method for upping the odds of finding their prey.
In a study published today in the Proceedings of the National Academy of Sciences—the first study to officially emerge from the VII—the researchers describe how they prepared a biochip that emulates the mucosal surface of a human lung epithelial cell. The biochip features lifelike secretion with the outermost mucus layers constantly sloughing off. Into the mucus on these biochips, they introduced E. coli bacteria and one of two types of phages: a normal, mucus-adherent E. coli–targeting bacteriophage known as a T4 phage (sticky phage, for this purposes of this article), or a genetically modified version of the phage that had lost its ability to stick to mucus (non-sticky phage). They also performed a control experiment in which they added bacteria to the mucus but not any phages whatsoever.
Barr and his colleagues observed how well the bacteriophages fared in finding and killing bacteria in the biochip’s mucus. The genetically modified, non-sticky phage, didn’t do any better than the control experiment. But the normal, sticky phages had “a really drastic antimicrobial effect,” Barr said, providing more than a 4,000-fold reduction in bacteria.
Stick to it
To see whether the normal phages were better than their non-sticky counterparts at persisting in the churning mucosal surface, the researchers performed another series of experiments designed to count phage accumulation and turnover. In the end, they found that the mucus expelled just as many normal phages as the non-sticky ones, meaning something other than mucus adherence must account for the difference.
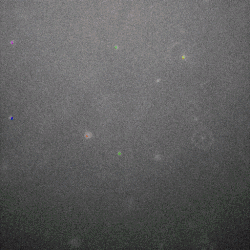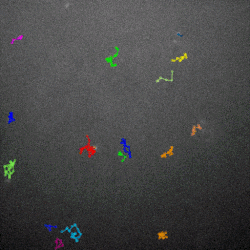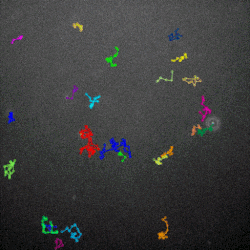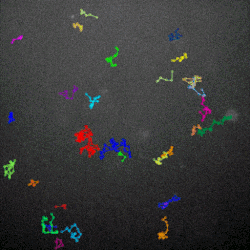
The researchers then used high-powered microscopes to watch how the phages diffused through different mucus environments. They found that the normal, sticky phage moved around in a more staccato fashion, lingering in one place for a little while, then moving to the next spot, and so on. That is known as subdiffusive motion, and the sticky phages in this experiment were able to accomplish it by weakly adhering to the mucus as they drifted.
Why would subdiffusive motion turn these phages into superior hunters? The answer, Barr said, lies in their prey’s abundance. When bacterial concentrations are high, random motion as a hunting strategy works just fine, he explained.
“If you’re in a mucus layer with really dense bacteria, you’ve got a pretty good chance of finding a host with random motion,” Barr said. “You can’t help but run into them.”
Slow your roll
When bacteria are scarce in the environment, or if there are a lot of different types of bacteria in abundance, phages that move in a slow, punctuated fashion are statistically more likely to encounter the right kind of bacterial prey.
“When bacterial diversity is high, it becomes difficult for a phage to find its specific host,” Barr said. “Under these conditions it’s more advantageous to move subdiffusively, to keep yourself in a location longer and seek out your prey more slowly.”
This makes bacteriophages unique among predators on earth, he added. It’s the first time an organism has been shown to use subdiffusion as a hunting strategy.
Researchers looking at phage therapy might one day be able to genetically modify bacteriophages to tweak their hunting strategies in order to maximize the chances they’ll encounter harmful bacteria, thereby reducing infection and disease. To that end, he and his colleagues have worked with SDSU’s technology incubator, the Zahn Innovation Center, to submit several provisional patents on this technique.
“There’s a huge amount of potential for ways you can engineer and optimize the system,” Barr said.
This research was supported by grants from the National Institutes of Health, the Gordon and Betty Moore Foundation, and internal funding from San Diego State University.



