Fighting Barnacle Buildup with Biology
New research by Nick Shikuma solves a mystery behind the gunk that sticks to the bottoms of boats.

“Biofouling is an economic issue.”
The coating of barnacles and other growth along the bottoms of boats is more than just an eyesore. Biofouling, as it is known, slows down ships and impedes the readiness of emergency response and military vessels.
“Biofouling is an economic issue,” said San Diego State University biologist Nick Shikuma.
A new study by Shikuma identifies key developmental steps these waterborne organisms must take to metamorphose from their larval to adult state. Understanding this process could lead to new technologies to prevent the organisms from attaching to ships in the first place.
Shikuma studies the life cycles of sea creatures like barnacles, sea squirts, urchins and tubeworms. A unique feature of these organisms is their reliance on a bacterial cue present in the environment to trigger metamorphosis. The specific bacteria they interact with have tiny, spear-like appendages that shoot out into the cells of the sea organisms and spur their metamorphosis. It’s during this metamorphic stage that the organisms settle upon a surface like a ship bottom.
“Bacteria-induced metamorphosis has been known to happen for almost a century, but no one knows how it works,” says Shikuma, a member of SDSU’s Viral Information Institute. The institute, led by biologists Forest Rohwer and Anca Segall, is at the forefront of investigations into microbial genomics.
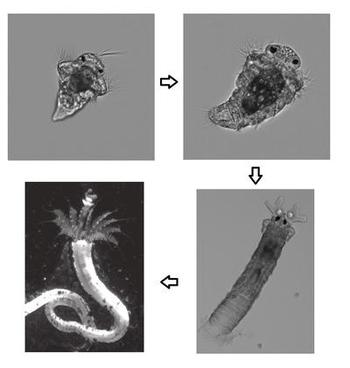
Hoping to learn more, he and colleagues sequenced the genome of the tubeworm Hydroides elegans, a frequent biofouling offender that leaves behind calcium carbonate tubes that stick to boats. To see which genes were active during metamorphosis, Shikuma introduced P. luteoviolacea bacteria to the tubeworms to kick off their metamorphosis and then analyzed the worms’ gene expression across different stages of its development.
The team found that a particular chain of proteins known as the MAPK signaling pathway is activated during metamorphosis. Did the bacteria spur the genes responsible for this process? To find out, the researchers repeated the experiment using genetically modified versions of P. luteoviolacea, some with functioning spear appendages and some with malfunctioning ones.
When the larval tubeworms were exposed to the bacteria with malfunctioning spears, their MAPK pathways showed less activation. The larvae began the initial stages of their metamorphosis but failed to complete the process, eventually reverting back to a larval state.
In other words, a normal interaction between the bacteria’s spears and the tubeworm’s MAPK pathway appears necessary for the tubeworms to successfully reach their adult stage. The researchers received funding from the U.S. Office of Naval Research for the work and published their findings in the Proceedings of the National Academy of Sciences.
Shikuma suspects similar organisms like barnacles and urchins might rely upon the same interaction with bacteria to complete their lifecycles. If so, researchers may one day be able to develop a ship coating with biological properties that inhibit the bacteria’s metamorphosis cue or the organisms’ MAPK pathways. Other potential applications include aiding the husbandry of marine animals for aquaculture or stimulating coral reef growth in areas decimated by reef loss.
On a broader scale, Shikuma’s findings elucidate the complex, intricate and largely understudied interactions between animals and bacteria. There are likely many other developmental processes and health effects influenced by these interactions—in humans as well as other animals—that have yet to be discovered.
“Tubeworms serve as a model organism to understand how bacteria can orchestrate the dramatic development of animals,” Shikuma said. “It's largely unknown how the interaction between bacteria and animals leads to normal development, health and wellbeing.”



